Synthesis, oligomerization and catalytic studies of a redox-active Ni4-cubane: a detailed mechanistic investigation†
Abstract
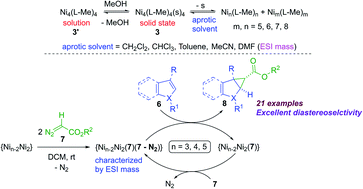
A robust tetrameric nickel complex [Ni4((Oal−)2L-Me)4(s)4] (3) (s = solvent) with cubane-like Ni4O4 core topology was isolated as a light greenish-orange crystalline solid in excellent yield. The mechanism of formation of 3 involving the two chloride-containing precursors [Ni4((Oal−)2L-Me)4(s)4]·2MeOH (1) and [Ni4((O−)2L-Me)3((Oal−)(OH)L-Me)Cl] (2) was studied by ESI mass spectrometry and confirmed by the solid state isolation and single-crystal X-ray diffraction. The challenging ligand fields containing mono/di-anionic O2N donating atoms and/or chloride ions stabilized the pentacoordinate Ni(II) ions in 1–2 upon controlling the experimental conditions. Complexes 1–3 have been characterized by NMR, UV-Vis and mass spectrometric analysis. Complex 3 was found to be redox active by cyclic voltammetry (CV) studies. Theoretical calculations were carried out to shed light on the effects of ligand fields on the stability of complexes 1–3. Complex 3 was found to be a potential catalyst for the diastereoselective cyclopropanation of heteroarenes with good to excellent yields. The ESI mass spectrometric analysis revealed the existence of solution dynamics and oligomerization of 3 in solution. Mechanistic investigation of the catalytic cycle revealed that complex 3 and its various oligomers bind to the diazoester employed, followed by dissociative insertion of the respective carbene moieties to the C2–C3 double bond of the involved aromatic heterocycle, leading to the diastereoselective cyclopropanation.


 Please wait while we load your content...
Please wait while we load your content...